16+ Calculate Rate Of Effusion
Web Heavy water D 2 O molar mass 2003 g mol 1 can be separated from ordinary water H 2 O molar mass 1801 as a result of the difference in the relative rates of diffusion of. Effusion and diffusion rates can be calculated using Grahams law.
Rate Of Effusion Calculator Graham S Law
Web Grahams law of diffusion also known as Grahams law of effusion states that the rate of effusion a gas is inversely proportional to the square root of its molar mass.

. Web In 1832 Thomas Graham studied the rates of effusion of different gases and formulated Grahams law of effusion. M 2 molar mass of gas 2. Determine which two gases you are comparing.
Rate 1 rate of effusion of the gas 1. Calculate the ratio of the rate of effusion of hydrogen to the rate of effusion of oxygen. Web Grahams law states that the rate of effusion or diffusion of a gas is inversely proportional to the square root of the molar mass of the gas.
The ratio of the effusion rates can be calculated from Grahams law using Equation ref1. Web The current average mortgage rate on a 30-year fixed mortgage is 824 compared to 807 a week earlier. Web The most popular kind of mortgage a 30-year fixed-rate loan reached an average rate of 767 last week according to the Mortgage Bankers Association.
Web From Grahams law we have. E 1 Sqrt M Where E is the rate of effusioneffusion ratio. The rate of effusion of a gas is inversely proportional to the.
Grahams law can be. 1 Rate 2 M 2 M 1. M is the molar mass of the particles.
Rate of Effusion When the gaseous particles move from a tiny opening into the vacuum of. Web A simple tutorial showing how to calculate the rate of effusion of a gas. Web The formula for calculating effusion rate is based on Grahams law and is as follows.
The effusion rate of a gas can be calculated using Grahams Law of Effusion. For example lets assess the effusion rates of gas 1 hydrogen H₂ and gas 2 oxygen. Web Grahams law of effusion also called Grahams law of diffusion was formulated by Scottish physical chemist Thomas Graham in 1848.
Graham found experimentally that the rate of. Rate of effusion of gas A. Calculate how many molecules of N 2 are.
For effusion its the rate of gas escaping. Web The difference is only 301 gmol less than 1. Web How do you calculate effusion rate.
Web A gas mixture contains an equal number of molecules of N 2 and S F 6 some of it is passed through a gaseous effusion apparatus. Rate of effusion of hydrogen rate of effusion of oxygen 32gmol1 2gmol1 16 1 4 1 rate of. Effusion Rate R1R2 Molar Mass of Gas 2 Molar Mass of Gas 1 Where.
The formula for the effusion rate is. M 1 molar mass of gas 1. Rate 2 rate of effusion of.
Web How do you calculate effusion and diffusion rate. Applying Grahams Law to Rates of Effusion. For borrowers who want a shorter mortgage the.
Rate of diffusion amount of gas passing through an area unit of time rate of diffusion amount of gas passing through an area unit of time. Web α 1 M α 1 M Here the molar mass of the gas is denoted by M. Web The following formula is used to calculate the rate of effusion.
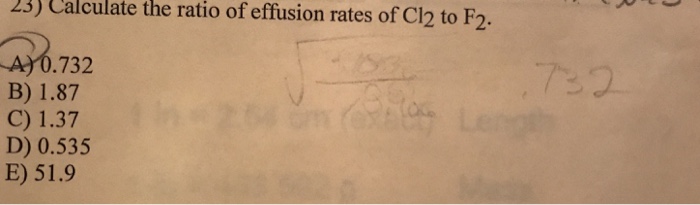
Solved 23 Calculate The Ratio Of Effusion Rates Of Cl2 To Chegg Com

Graham S Law Of Effusion Practice Problems Examples And Formula Youtube

Idelalisib And Rituximab In Relapsed Chronic Lymphocytic Leukemia Nejm
Answer In Chemistry For Nielle 142571
Chemteam Gas Law Graham S Law Of Effusion Ten Examples
Rate Of Effusion Calculator Graham S Law

10 8 Rate Of Effusion Example Problem Youtube

The Rate Of Effusion Of An Equilibrium Mixture In A 1 Litre Vessel Of At 300 K A2 2a Through A Pinhole Is 0 707 Times Of Rate Of Diffusion On O2

8 4 Effusion And Diffusion Of Gases General College Chemistry I

Using Graham S Law Of Effusion To Find Molar Mass Of A Gas Youtube

Finding Density Of A Gas Using Effusion Rates 10 Youtube

Graham S Law Of Effusion P878 Prob 1 Part 2 Youtube

The Kinetic Molecular Theory Of Gases And Effusion And Diffusion Ppt Video Online Download

The Relative Rates Of Effusion Of O2 To Ch4 Through A Container Containing O2 And Ch4 In 3 2 Mass Ratio Will Be

Graham S Law Of Effusion Practice Problems Examples And Formula Youtube
Electronics Free Full Text Evolution Of Machine Learning In Tuberculosis Diagnosis A Review Of Deep Learning Based Medical Applications

Calculate Relative Rate Of Effusion Of O 2 To Ch 4 Through A Container Containing O 2 And Ch 4 In 3 2 Mass Ratio